Let’s discuss the question: how to draw an ion. We summarize all relevant answers in section Q&A of website Achievetampabay.org in category: Blog Finance. See more related questions in the comments below.
How do you make ions?
Ions are formed by the addition of electrons to, or the removal of electrons from, neutral atoms or molecules or other ions; by combination of ions with other particles; or by rupture of a covalent bond between two atoms in such a way that both of the electrons of the bond are left in association with one of the …
What is the ion of oxygen?
Oxygen, O. Oxygen is in Group 6. It has six electrons in its outer shell. It gains two electrons from one or two other atoms in reactions, forming an oxide ion, O 2–.
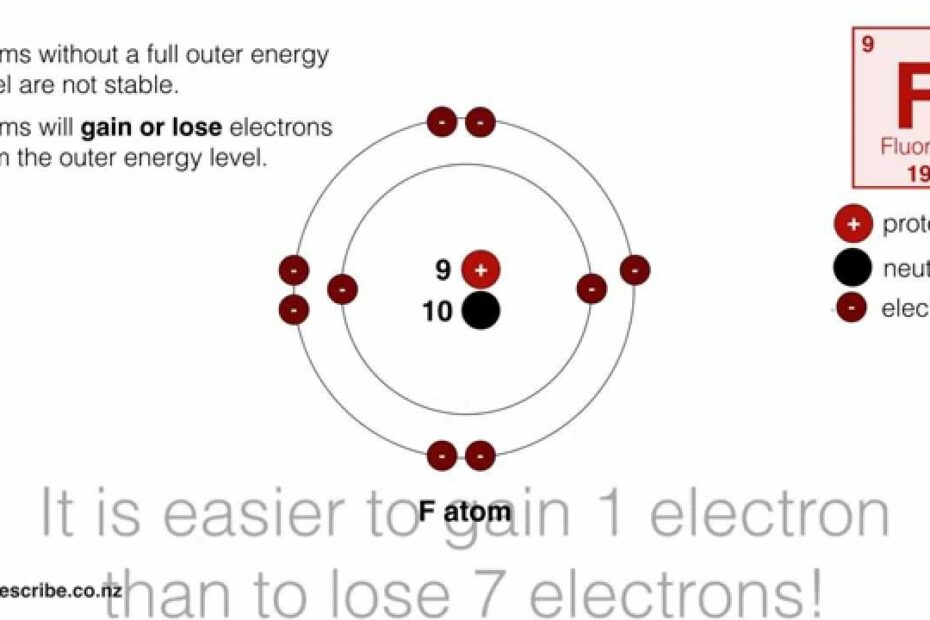
Drawing Ions (NCEA L1 \u0026 Junior Science)
Images related to the topicDrawing Ions (NCEA L1 \u0026 Junior Science)

How do you draw a atom?
- Begin by drawing a circle. This will form the atom’s nucleus. …
- Draw a smaller circle some distance away from the first circle. This forms the first electron. …
- Draw an incomplete oval around the nucleus. …
- Draw another curved line roughly parallel to the first.
What does an anion look like?
…
Cation vs anion chart.
| Cation | Anion | |
|---|---|---|
| Charge | Positive | Negative |
| Electrode attracted to | Cathode (negative) | Anode (positive) |
| Formed by | Metal atoms | Non-metal atoms |
What is ion example?
Gaining electrons makes the atom negatively charged, and losing electrons makes the atom positively charged. Positive ions are called cations, and negative ions are called anions. For example, lithium loses 1 electron to become Li+1. The +1 indicates the ion has a +1 charge.
What is ion in physics?
An ion (/ˈaɪɒn, -ən/) is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention.
How do you find ions?
When writing the symbol for an ion, the one- or two-letter element symbol is written first, followed by a superscript. The superscript has the number of charges on the ion followed by a + (for positive ions or cations) or – (for negative ions or anions). Neutral atoms have a charge of zero, so no superscript is given.
Is NaCl ion or molecule?
Something like table salt (NaCl) is a compound because it is made from more than one kind of element (sodium and chlorine), but it is not a molecule because the bond that holds NaCl together is an ionic bond. If you like, you can say that sodium chloride is an ionic compound.
Review Drawing Atoms and Ions
Images related to the topicReview Drawing Atoms and Ions

How are ionic compounds formed?
An ionic bond is formed by the complete transfer of some electrons from one atom to another. The atom losing one or more electrons becomes a cation—a positively charged ion. The atom gaining one or more electron becomes an anion—a negatively charged ion.
How are ionic compounds structure?
An ionic compound is a giant structure of ions. The ions have a regular, repeating arrangement called an ionic lattice . The lattice is formed because the ions attract each other and form a regular pattern with oppositely charged ions next to each other.
What is the electron dot diagram for lithium?
…
Electron Dot Diagrams.
| lithium | 1 s 2 2 s 1 | 1 valence electron |
|---|---|---|
| neon | 1 s 2 2 s 2 2 p 6 | 8 valence electrons |
How many dots belong in the electron dot diagram?
By going through the periodic table, we see that the Lewis electron dot diagrams of atoms will never have more than eight dots around the atomic symbol.
What is ion formation?
Ions are formed when atoms lose or gain electrons in order to fulfill the octet rule and have full outer valence electron shells. When they lose electrons, they become positively charged and are named cations. When they gain electrons, they are negatively charged and are named anions.
What ion is carbon?
| Element name | Ion name | Ion formula |
|---|---|---|
| Sulfur | Sulfide | S2− |
| Nitrogen | Nitride | N3− |
| Phosphorus | Phosphide | P3− |
| Carbon | Carbide | C4− |
What ion is ba2+?
Barium ion | Ba+2 – PubChem.
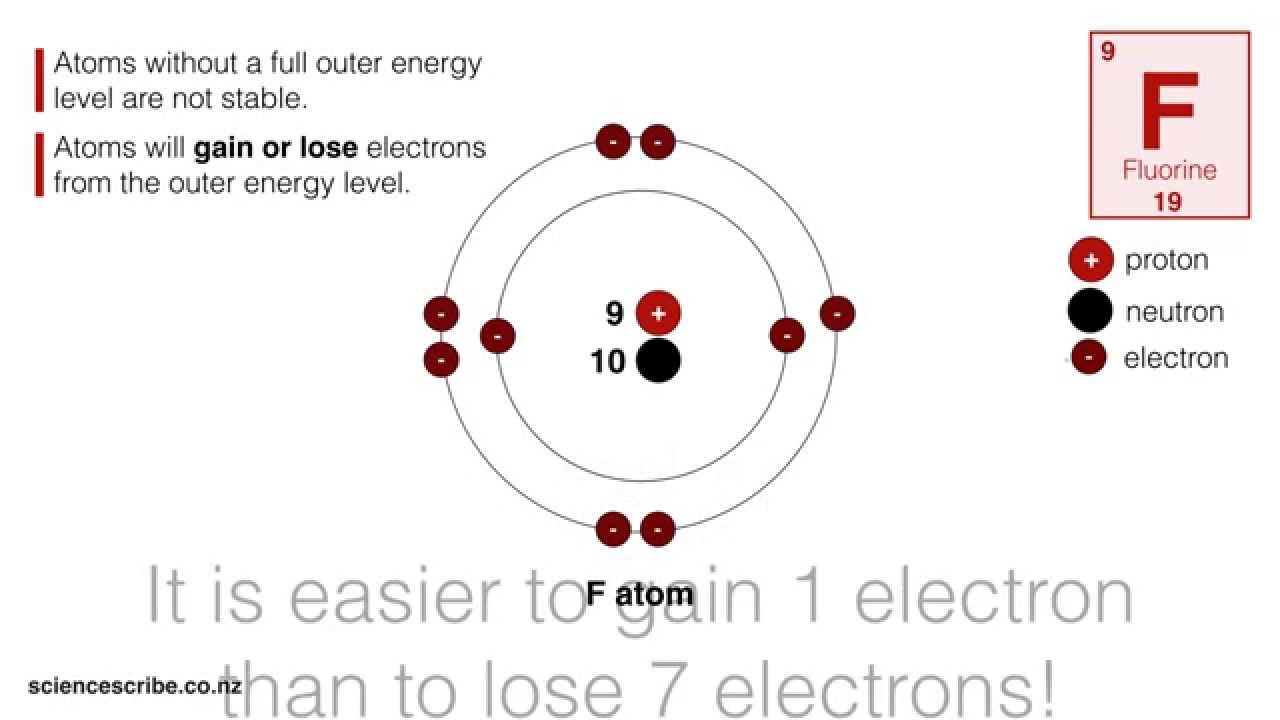
How to Draw an Ion
Images related to the topicHow to Draw an Ion

How do you draw electrons in an atom?
- Find the element on the periodic table. …
- Draw a small circle and write the symbol in the centre. …
- Draw a circle around the nucleus. …
- Add up to two electrons to the first electron shell. …
- Draw another circle around the first shell. …
- Add up to eight electrons to the second shell.
How do you draw a helium atom?
Draw a circle about 2 inches in diameter on a piece of paper. The circle represents the nucleus of a helium atom. Add two “+” symbols inside the circle to represent the two positively charged protons in a helium atom’s nucleus. Draw two small zeros inside the circle to represent the two neutrons in the nucleus.
Related searches
- how to draw an ionic lattice
- how to draw a hydrogen ion
- ionic bond drawing
- sodium ion
- anion drawing
- lithium-ion
- how to draw an ionic bond lewis structure
- how to draw an ionic bond
- how to draw a bohr rutherford diagram for an ion
- draw me an ion
- how to draw a lewis dot structure for an ion
- how to draw an atom
- how to draw an ionic solid
- lithium ion
- how to draw an ionic compound
- how to draw an ionized calcium
- how to draw an ionic column
- how to draw a lewis structure for an ion
- how to draw an ion bohr diagram
- sodium-ion
- how to draw an ion diagram
- sodium ion drawing
- al draw ion
- how to draw a lewis dot diagram for an ion
Information related to the topic how to draw an ion
Here are the search results of the thread how to draw an ion from Bing. You can read more if you want.
You have just come across an article on the topic how to draw an ion. If you found this article useful, please share it. Thank you very much.